HTI-1 update for USCDI v3 and (g)(9)
Your EHR Needs USCDI v3 by Jan 1, 2026. Here’s What it Means.
USCDI v3, (g)(9), and HTI-1 Deadlines
From last week’s White House announcement, the message is clear: patient-centered, interoperable healthcare is not a distant vision, but a current need. For EHR vendors, that translates into one thing: USCDI v3 is non-negotiable. As of Jan 1, 2026, certified health IT must support USCDI v3 for all criteria requiring a C-CDA (Consolidated Clinical Document Architecture) reference. This blog focuses on one crucial criterion - (g)(9) Application Access - All Data Request, which influences patient data sharing using third-party apps. There are no extensions, no delays.
We are already in Q3 2025. So, if your EHR isn’t ready, now’s the time to act.
Let’s examine what this update means for your EHR and how Darena Health can make it easier to implement it.
(g)(9) Requires All the Data, On Demand
The (g)(9) criterion requires that your EHR let patients access all their data for a specific encounter. This is not just a handful of fields like medications or allergies; that is what (g)(10) does. (g)(9) requires the certified EHR to deliver all data fields defined in USCDI v3, in a way that apps can use.
What this means: If a patient uses an app to request their complete encounter data, your EHR needs to deliver the full dataset in a structured, machine-readable format in a timely fashion without any special effort from the patient. If you don’t already support all data elements of USCDI v3, this task must be completed by December 31, 2025, which is challenging.
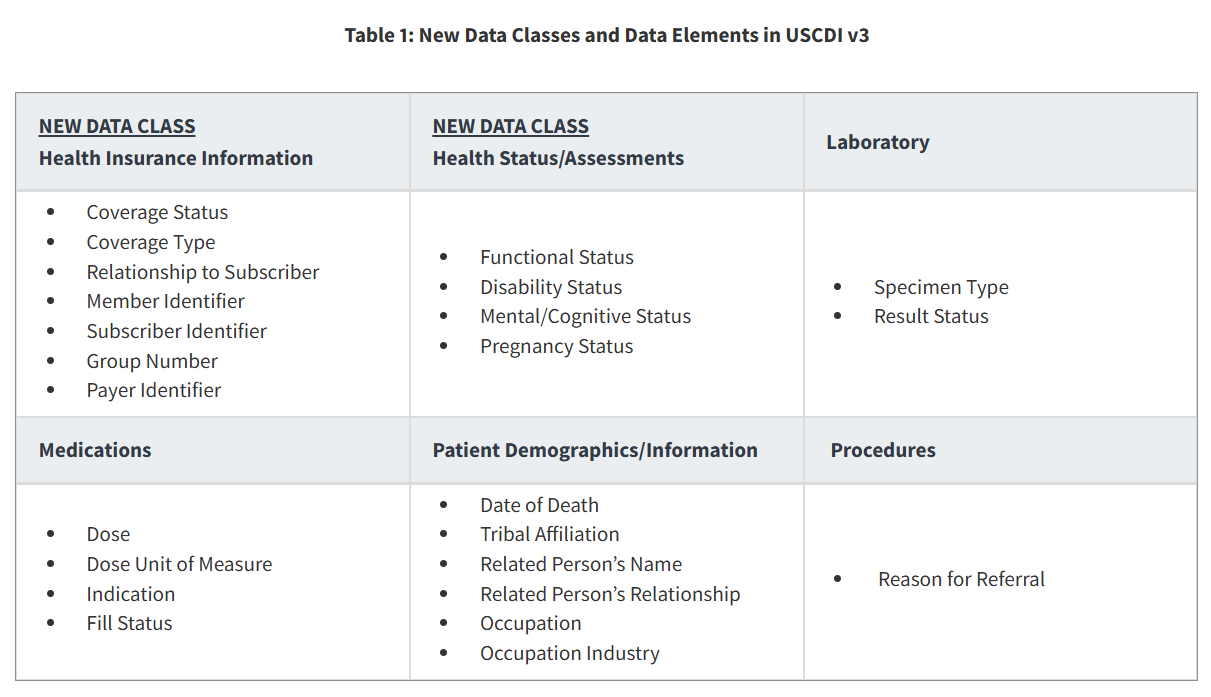
What is new with USCDI v3? Why now?
Source: ASTP Standards Bulletin 2022-2 | HealthIT.gov
USCDI v3 is the version of the United States Core Data for Interoperability that EHRs are required to support by January 1, 2026, as specified in the HTI-1 Final Rule. It sets the national standard for what types of clinical data should be shared.
USCDI v3 represents a significant increase in the data elements as ASTP/ONC went from requiring v1 to v3; the update contains USCDI v2 and USCDI v3 data elements. Health Insurance Information, SDOH, and refined demographics are a few examples of new complex data elements, making the update to USCDIv3 a heavy lift for EHR developers.
To be compliant with (g) (9), your EHR must support all the old and new USCDI data classes and data elements without missing any fields.
The Usual Approach for (g)(9): Send a C-CDA
The most common way EHRs try to meet the (g)(9) requirement is by generating a C-CDA. This structured document can include many types of patient data.
As of January 1, 2026, when a patient or app requests their complete encounter data, the EHR will need to send a C-CDA that includes the appropriate USCDI v3 data.
Challenges with C-CDAs for EHRs
As USCDI keeps expanding, many EHR vendors are struggling with:
Updating C-CDA templates: C-CDA templates were not designed for the newer fields in USCDI v3. C-CDA can still be used for (g)(9), but it requires careful updates to support USCDI v3 fully. This means that even a minor omission or formatting issue could make your export incomplete and thus non-compliant.
Certification attestations: These add to the certification maintenance tasks for EHRs.
Managing app interoperability: As the C-CDA data elements expand, all the fields need to be supported to continue information sharing and avoid information blocking. Trying to patch things as you go adds the risk of non-compliance.
How Darena Makes it Easy
Darena Health offers a better way to meet the (g)(9) requirement for all USCDIv3 data elements without overloading your development team. You have two choices:
1. Keep Using Your C-CDA
If you want to continue generating C-CDAs, Darena will accept them. However, you’ll be responsible for ensuring they include every USCDI v3 field. Any missing data must be sent separately using FHIR.
2. Use Darena’s FHIR API (Recommended)
You can defer to our (g)(9) certified module using this approach [similar to (g)(10) FHIR API]. This new capability will handle all the underlying certification requirements, including ongoing USCDI version updates when required, so you don't have to manage the complexities of mapping and transformation.
We are making changes to our APIs, which will enable you to upload simple JSON-formatted data directly to us, and we will return a valid USCDIv3 C-CDA. You send us structured data. We take care of formatting, validation, and compliance.
This updated C-CDA will also satisfy the USCDIv3 requirements for the following criteria:
§170.315(b)(1) – Transitions of Care
§170.315(b)(10) - Electronic Health Information Export (Available as a Certified Module)
§170.315(b)(11) - Decision Support Interventions (Available as a Certified Module)
§170.315(b)(2) – Clinical Information Reconciliation
§170.315(e)(1) – View, Download, and Transmit
§170.315(f)(5) – Electronic Case Reporting
§170.315(g)(6) - Consolidated CDA Creation
§170.315(g)(9) – Application Access – All Data Request (Will soon be available as a Certified Module)
§170.315(g)(10) - Standardized API for Patient and Population Services (Available as a Certified Module)
Why More EHRs Are Choosing Darena
At Darena Health, we align with ASTP/ONC and CMS’s mission to create a more connected, patient-centered healthcare ecosystem. With the January 1, 2026, deadline for USCDI v3 fast approaching, many EHR vendors are facing increasing pressure to adapt to complex technical and regulatory demands. That’s where we come in.
We specialize in helping health IT developers meet certification requirements over the years, with minimal disruption to their existing architecture. Our solutions are designed to reduce the workload on your engineering, compliance, and development teams without compromising quality or control.
Here's what sets Darena apart:
Faster compliance: We keep up with ASTP/ONC rules, so you don’t have to.
Simplified implementation: No need to manage C-CDA templates or custom FHIR mappings for multiple criteria.
Data done right: Fewer gaps, more complete records for your customers.
Certifications covered: One integration gives you access to certified modules supporting (g)(6), (g)(7), (g)(9), (g)(10), (b)(10), and (b)(11) with seamless expansion as needed.
Partnering with Darena means having a dedicated HITRUST-certified team focused on compliance, interoperability, and long-term adaptability without adding new build cycles or regulatory complexity to your roadmap.
Final Thoughts
USCDI v3 and (g)(9) are not just regulatory checkboxes. They shape how patients access and control their health data. Falling short of the new expectations could mean failed certifications, information blocking, and customer attrition.
Darena helps you satisfy production requirements and stay compliant without the hassle. Whether you stick with your current C-CDA approach or use our JSON API, you’ll get full USCDI v3 support and seamless (g)(9) coverage.